HBOT
Hyperbaric Oxygen Therapy
is a clinical treatment where the patient breathes 100% oxygen intermittently while enclosed in a hyperbaric oxygen chamber at a pressure greater than one atmosphere.
Hyperbaric Oxygen Therapy is growing fast and continuously. More than 17 diseases have been approved to be treated by Hyperbaric Oxygen treatment in Europe and USA.
WHY IS OXYGEN SO IMPORTANT?
Oxygen is colourless, odourless gas that makes up about 21 percent of the atmosphere. It is essential to life for two reasons :
- Oxygen is one of the body's basic building blocks. All of the body's major components - water, protein, fat and carbohydrate contain oxygen.
- Oxygen helps bring about certain chemical reactions within the body that result in energy production.
How does HBOT affect our Body?
- Decreases Inflammation
- Saturates the body with oxygen, Increasing the level by 20-30%.
- Increases the body's immunity.
- Creates new capillaries and increases blood flow.
- Clears and deactivates toxins and metabolic waste from the body.
- Stimulates the body to create new blood cells
- Increases the body's production of stem cells 800% (after 40 treatments)
- Accelerates the rate of healing
- It reduces swelling

O2multi HBO CHAMBER
O2multi type Multiplace HBO Chambers are produced in different sizes from 4 Person up to 12 Person. If requested we can produce more than 12 person chambers. The Multiplace Hyperbaric Treatment System is designed to provide hyperbaric oxygen therapy to patients at pressure up to 5.5 bar as well as treating diving accident. The entire hyperbaric treatment system including all subsystems, components, and ancillary support systems are designed to meet or exceed stringent hyperbaric engineering design and fabrication standards for health care facilities as required by EN14931 “Pressure vessels for human occupancy (PVHO) – Multiplace Chambers”.
The Multiplace treatment system is designed to pressurize the chamber using compressed medical grade air. Hyperbaric oxygenation is achieved by providing the patient 100% pure oxygen by mask or hood while exposed to elevated barometric pressure. Technical specifications of our O2multi chamber;

HBO CHAMBER (PRESSURE VESSEL)
Baroks O?multi Multiplace Chamber is designed manufactured and tested according to EN 14931 norms. Pressure Vessel consists of 2 compartments which are called Main compartment and Entry compartment. Main compartment capacity is 12 patients and 1 health official and entry compartment capacity is 2 patients.
Dimensions (12+2 Persons)
The main compartment and entry compartments are 2,200 mm internal diameter with a combined overall length of 6,500 mm. The main compartment has an internal length of 5,000 mm and the entry compartment is 1,200 mm long. All dimensions are approximate within standard fabrication tolerances.
Painting
Chamber is painted with certified flame-proof and non-poisonous marine type epoxy paint both inside and outside. In order to make chamber surface smooth, sand blasting process applied to surfaces and then pasting is applied to the surface.
Materials and Tests
Only quality-certified “Boiler Steel H-II” with test report is used in O2multi Multiplace Chamber production. In addition; before the production, ultrasonic lamination tests and after welding process; xray, magnetic particle and ultrasonic tests (Non-destructive tests) are done by engineers. After the production, hydrostatic tests are done by engineers with attendance of TUV inspectors.
Medical Lock
Medical Lock provides the transfer of the necessary medicines or equipment’s to and from the chamber during a session without interruption. Medical Lock is equipped with a double secured lock system and the accidental opening of the outer door of the medical lock is prevented when the chamber is pressurised.
Doors The chambers are designed to have 3 doors. Doors are designed as rectangular to allow easy access to the chamber interior. Regular sizes are 1,760mm X 860mm (Clear Height X Width), however can be designed and produced bigger if requested.
Viewports
Viewports are used to observe the patients from outside. 6 viewports are used in main compartment and 2 in entry compartment.
CHAMBER EQUIPMENTS
Chamber equipments involve equipments in or on the chamber itself. These equipments are listed as minimum as follows:
BIBS (Built-in Breathing System)
Chamber BIBS system provides the required oxygen for the breathing of the patients and health official inside the chamber and also discard the left-over air with exhaust system. Oxygen Treatment Hood is provided to administer oxygen or other therapy gases to patients as well as the mask. O2multi Multiplace Chamber is equipped with AVOX BIBS regulators for patients
Emergency Valves Emergency Discharge Valves keeps the inside chamber not to exceed settled maximum level. When the chamber pressure increases too much it discharges the excessive pressure to prevent patients from getting harm. There are two types of valves in the system. Automatic valves initiates discharging the inside air as soon as the chamber pressure reaches 3 bar. Manual valves are used when the automatic valves are out of order or when speeding up of the discharge is needed. All emergency valves are CE certified.
 Seats
Seats O?Multi Multiplace seats are easily removable and orthopaedic just made specifically for hyperbaric use. The seats are designed in such a way to ease the assembling and dismantling.
Internal LED Lighting System Internal lighting of the chamber is provided by 12V LED light source. This provides safety fire protection against any electrical sparks. The switches are on the control panel.
Sensors There are sensors in and out of the chamber which are Pressure, temperature, humidity and oxygen sensors, etc. All sensors are CE marked and approved for use in hyperbaric environment. There are 2 pressure sensors; one in the main and one in the entry compartment.
CCTV Cameras O?Multi chambers are equiped with high quality lens cameras for each compartment. It also contains infrareds to enable observation even in zero lux condition. There are four cameras installed in the main compartment and 2 in the entry compartment. All patients can be monitored during the session.
Entertainment TV Monitors Baroks O?Multi Multiplace chambers are equipped with DVD entertainment system. There are 2 pieces 21” LCD 12V DC monitors are installed on the doors.
Internal Information Panel There is information panel inside the chamber. Patients can observe the temperature, humidity, pressure and oxygen level through this panel.
MANUEL CONTROL CONSOLE
Control Panel Baroks Control Panel provides the use and control of the Multiplace Chamber System manually. Pneumatic Equipment (Attached to the Chamber) Baroks O?Multi contr ol panel attached to the chamber also includes following pneumatic equipments :
- Electric drive to control descent rate (compression)
- Electro-pneumatic proportional rate valve.
- Manuel bypass handles for compression.
- Electric drive to control ascent rate (decompression)
- Electro-pneumatic proportional rate valve
- Manuel bypass handles for decompression.
- Decompression valve
- 1x LP air c/W gauge (main supply)
- Pipes connections, fittings for
- 2-solenoid valve to switch the BIBS from oxygen to air.
- Valves for fire suppression deluge system 2 x 6” mirror scale (0-230msw) precision debt gauges


MAIN CONTROL PANEL
Automation panel in O?multi Multiplace Chamber Systems has been developed specially for automatic control of chamber system and designed ergonomically for operator use
The panel consist of the following equipments:
- Set of light dimes for illumination of MC and AC
- Patient (audio/visible) warning signals
- Intercom (electrical communication) system for AC & MC with volume control, earphone and microphones and PTT function
- Sound powered phone (SP-T)
- PLC (automation) control for compression and decompression
- Cooling heating control switches
- Fire suppression controls (controls for water deluge for fire suppression)
- Gas selection (air/oxygen) for the Breathing system for AC & MC
- Fault warning system
-
- Electrical main power loss
- Oxygen concentration exceeding %19-%23
- Exceeded descent rate
- Exceeded ascent rate
- Oxygen supply failure
- Water deluge fire fighting reservoir failure
- Emergency warning for AC and MC
- Information panel digital readouts for actual time (24 hour/min)
- Oxygen concentration (%) AC and MC
- Chamber temperature
- UPS (30 min at least) for safety vital equipments such as illumination, gas analyzers, communications, monitoring, fault recording system

COMPUTER CONTROLLED SYSTEM
Baroks O?Multi Multiplace Chamber is controlled and observed from a Computer Controlled Station. Baroks O?Multi Multiplace Chamber is fully automatic with industrial based PLC system and automation software. Main features of the system are :
- Automation System (ISO 25051 Certified)
- Industrial PLC based PC
- Automation software
- Customized
- Generating and storage of numerous treatment profiles.
- Graphical display of pressure vs time, oxygen %, temperature, humidity, gas stock pressure.
- Separate display for AC and MC
- Treatment number, treatment for patient number
- Keyboard and mouse integrated with the panel
- Patient and session info register
- 3-step security encoding
- Fault feedback
- PC (512 GB hard disk, cordless keyboard and mouse)
- 17” colour TFT monitor
- Sound powered phone for AC and MC
- 2 x CO2 analyser feeds into PC system and controls the flush rate (ventilation).
- 2 x Oxygen analyser 0-30%. Analyzers feed into PC system and controls the flush rate (ventilation).

O? Mono Hyperbaric Chamber
The Baroks O?mono Model Monoplace Hyperbaric Treatment System consists of one compartment, is designed to safely provide hyperbaric oxygen therapy to patients at pressure up to 2 bar (3ATA). Monoplace chamber is designed according to European Standarts and is tested according to EN14931 requirements.
Only quality-certified “Special Steel” with test reports is used in our Monoplace Chamber production. In addition; before production of the chamber, ultrasonic lamination tests and after welding process; x-ray, magnetic particle and ultrasonic tests (Non-destructive tests) have been done by professional engineering staff. All these process is controlled and reported by certification company. After chamber production, hydrostatic tests is done by the certification authority surveyors.
The chamber has cylindrical shape and one entrance door
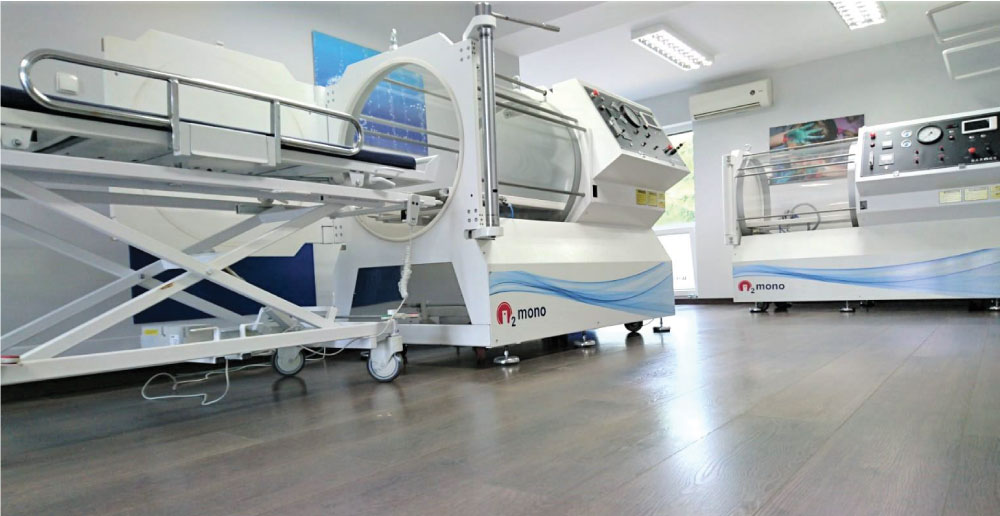
We are manufacturing two type monoplace chamber :
Half Acrylic (HA) and Full Acrylic (FA).
GENERAL VIEW OF TWO TYPES

MONOPLACE HBOT CHAMBER
Are designed to treat a single person pressurized with 100% oxygen.

CHAMBER CONSISTS OF BELOW MENTIONED PARTS
Table 1 : Chamber Parts

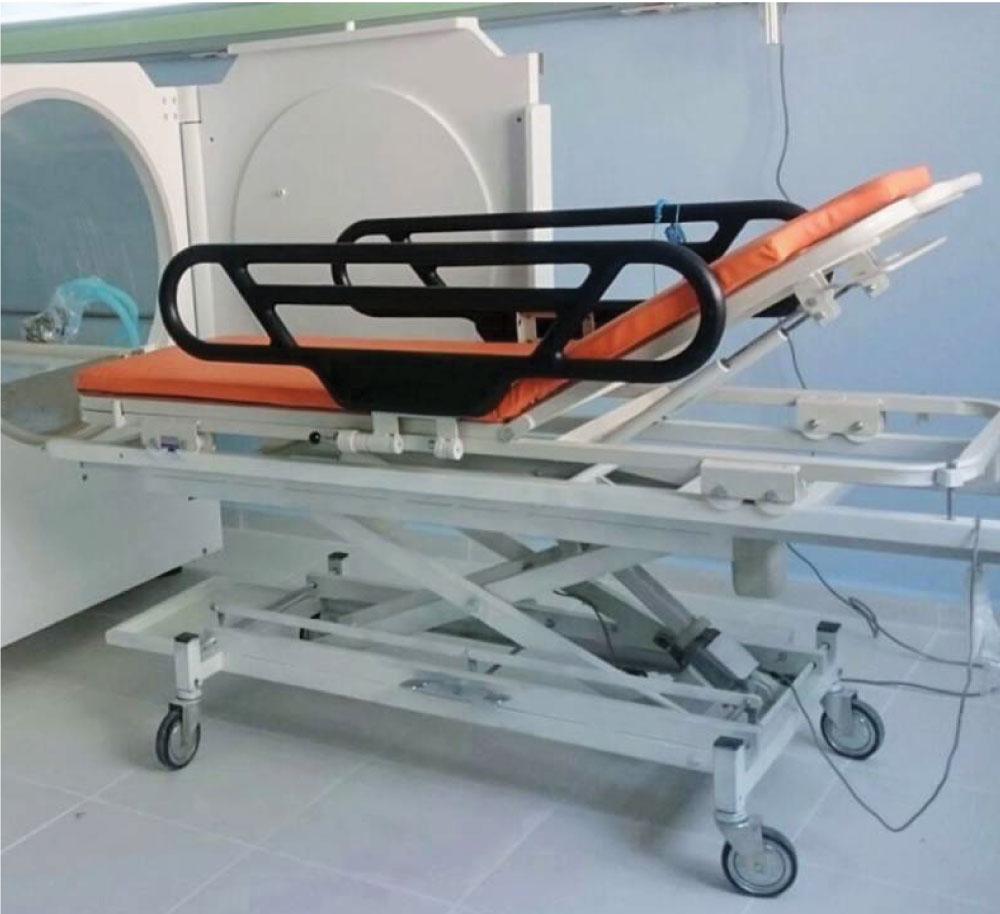
PATIENT TRANSFER UNIT
Stretcher is used for patient transferring and it consists of two parts as chamber internal stretcher and transfer stretcher. Internal stretcher is transported with transfer stretcher while the patient is transported into the chamber by pushing The stretcher is designed for working in hyperbaric conditions as mechanically.

O? Decompression Chambers
Following IMCA guidelines and using European standards (GL, DNV etc.) Baroks produce and outfit its chambers with highest quality equipment. Keeping ‘safety first’ always in priority we take all precautions during production of the chamber as imposed by the standards. Extensive range of optional equipments are also available to allow the system to be custom-designed.
WHY IS OXYGEN SO IMPORTANT?
Decompression Sickness (DCS) is a condition in which rapid changes of pressure in an environment causes gases to form bubbles of gas, mainly nitrogen. In diving, when the diver descends, nitrogen is breathed in and is dissolved in the blood and tissues. Subsequently, nitrogen leaves the blood and tissues and forms into tiny bubbles when the diver ascends due to the drop in the surrounding pressure. If the nitrogen is not re-absorbed into the blood and exhaled through the lungs, these tiny bubbles can form into larger bubbles and cause serious damage to your body.
A variety of DCS symptoms may then arise when these bubbles become large in number and block the flow of blood.
Things such as diver-related errors, misuse of dive tables, malfunctioning dive computers or inaccurate depth gauges, are also contributory factors leading to DCS.
WHAT ARE THE INSTANCES IN WHICH DCS MIGHT COMMONLY OCCUR?
There are three instances in which decompression sickness may occur:
- Divers who ascend too rapidly from a dive,
- Long-lasting or multiple dives,
- Divers who set off too early from low altitude (surface) to high altitude (mountain or air).
WHAT ARE THE SIGNS AND SYMPTOMS OF DCS?
The most common symptom of decompression sickness is pain in the arms or leg joints.
The other symptoms and signs that can be observed are :
- Blotchy skin rash
- Fatigue or weakness
- Skin itchiness
- Dizziness
- Shortness of breath
- Abdominal distress
- Chest pain
- Skin swelling
The above signs and symptoms may occur when the diver is still underwater, or they may come about as late as 36-48 hours after a dive.
HOW IS DCS TREATED?
Decompression sickness can only be treated through recompression in a high-pressure chamber. This apparatus slowly increases pressure on the person, forcing the bubbles to dissipate. The pressure is then gradually reduced to enable the person to breathe out the extra gases. Operation of the high-pressure chamber shall only be done by qualified medical personnel.
As soon as you suspect DCS, administer 100% oxygen through a tight-fitting mask. As soon as possible, seek for Emergency Medical Service (EMS) or the nearest medical assistance available.
TECHNICAL SPECIFICATIONS
The below table shows technical specifications about decompression chamber system.
| Working Pressure |
: |
6 bar |
| Test Over Pressure |
: |
9 bar |
| Main Chamber Patient Capacity |
: |
3 Persons |
| Chamber Diameter |
: |
1.700 mm |
| Length of Chamber |
: |
3.000 mm |
| Material |
: |
HII Steel |
| Number of Doors |
: |
2 Pcs. |
| Weight approximately |
: |
3500 kg |
| Door Diameter |
: |
800 mm |
| Windows on The Chamber |
: |
4 Pcs. |
| Windows Diameter |
: |
220mm ( Clear ) |
| Acceptance Society Pressure Vessel |
: |
TUV SUD |
| Product Standards |
: |
IMCA |

DECOMPRESSION CHAMBERS
Our products are manufactured in compliance with world’s top quality standards, which guarantee both patients and personnel comfort and safety. The company is managed by a financial group comprised of physicians and engineers specializing in the HBO business. For the purposes of subassembly and service procurement, we cooperate only with world-reputed manufacturers of medical, electronic and pressure equipment. Our company complies with global environmental quality standards. We render top quality maintenance services.Baroks O?Deco Decompression Chamber System is designed, manufactured and tested according to EN 14931 norms.
Chamber Design has been made according to AD2000. Pressure Vessel consists of 2 compartments which are called Main compartment and Entry compartment. Only quality-certified “Special Steel” with test reports is used in our Decompression Chamber production. In addition; before production of the chamber, ultrasonic lamination tests and after welding process; x-ray, magnetic particle and ultrasonic tests (Non-destructive tests) have been done by professional engineering staff. All these process is controled and reported by certification company. After chamber production, hydrostatic tests is done by the certification authority surveyours.
The chamber has cylindirical shape and one enterance door.

MATERIALS AND TESTS
Only quality-certified “Boiler Steel H-II” with test reports has been using in O?deco Decompression Chamber production. In addition; before production of the chamber, ultrasonic lamination tests and after welding process; x-ray, magnetic particle and ultrasonic tests (Non-destructive tests) have been doing by professional engineering staff. After the production, hydrostatic tests have been doing by engineers with attendance of TUV inspectors.
DOORS
In order to meet best comfort for the patients and operators, Baroks’ chambers are designed as two-door for MC and TC. Circular watertight door diameter is 800 mm
BIBS ( BUILT-IN BREATHING SYSTEM )
Chamber BIBS system provides the required oxygen for the breathing of the patients and health official inside the chamber and also discard the left over air with exhaust system. The chamber exhaust system provides the means of removing expired oxygen from the BIBS masks to outside to the chamber.

LIGHTNING SYSTEM
Hyperbaric approved LED lighting is used in O?Deco Decompression Chamber. The light assemblies are divided into groups. The lights generate no noise.
EMERGENCY VALVES
Emergency Evacuation (Relief) Valves provides to keep the inside chamber not to exceed settled maximum level. In a faulty case when the chamber pressure increases too much it evacuates the excessive pressure to prevent patients getting harm. There are two types of valves in the system. Manual valves are used when the automatic valves are out of order or when speeding up of the discharge is needed. All used emergency valves are CE certified best quality products from Europe.
EQUIPMENTS IN THE CHAMBER
- 3x Oxygen Breathing Systems (BIBS)
- 2x Orthopedic folding seats with back rest (with hardly inflammable certificate)
- 1x Bunk
- 4x Unit LED lighting
- 1x Intercom Unit
- 1x Manometer
- 1x Thermometer
- 2x Silencers
- 1x Analogue Clock
- 2x Fire extinguishers (water spray type) 9 lt
- 6x Sprinklers for fire suppression system
- 1x Stainless steel ball valve for drainage
- 1x Medical lock stainless steel
- 1x Safety valve
- 1x Door of 800 mm diameter
- 1x Pressure transmitter
- 1x Temperature transmitter
- 1x Bumidity transmitter
- 1x FANCOIL system for Aircondition
- 1x Aluminum Floor
















































